 Xiamen Baysen Medical Tech Co., Ltd. is a high-tech biological enterprise which devotes itself to the field of fast diagnostic reagent and integrates research and development, production and sales into a whole. Our factory devotes itself to developing new rapid diagnostic reagents and instruments to meet the market demand, continually innovating and enterprising and continuously improving the detection of diseases, Prevention and treatment level, to protect the lives and health of the general public. Our company is strictly following with
Xiamen Baysen Medical Tech Co., Ltd. is a high-tech biological enterprise which devotes itself to the field of fast diagnostic reagent and integrates research and development, production and sales into a whole. Our factory devotes itself to developing new rapid diagnostic reagents and instruments to meet the market demand, continually innovating and enterprising and continuously improving the detection of diseases, Prevention and treatment level, to protect the lives and health of the general public. Our company is strictly following with
I S O 1 3 4 8 5 and I S O 9 0 0 1 quality management system operation with research, production, quality control, international sales etc and has many advanced research staffs and marketing managers in the company, not only the quality management but also the serving. We win good reputation from abroad and domestic customers.
Companies adhere to the "people-oriented" concept of employment, respect and pay attention to the value of staff, and strive to build a good business environment, win-win values and management concepts to establish a more innovative, cohesive and competitive human resources system, and expect enterprises and employees develop together.
Abbott is our sole agent for some reagent in China, we are the first factory to register CFDA for calprotectin rapid test kit in China, the quality in China is also on the top.
Our mission is to be a whole solution provider of POCT products to live better for the whole world.
Get to know Baysen medical
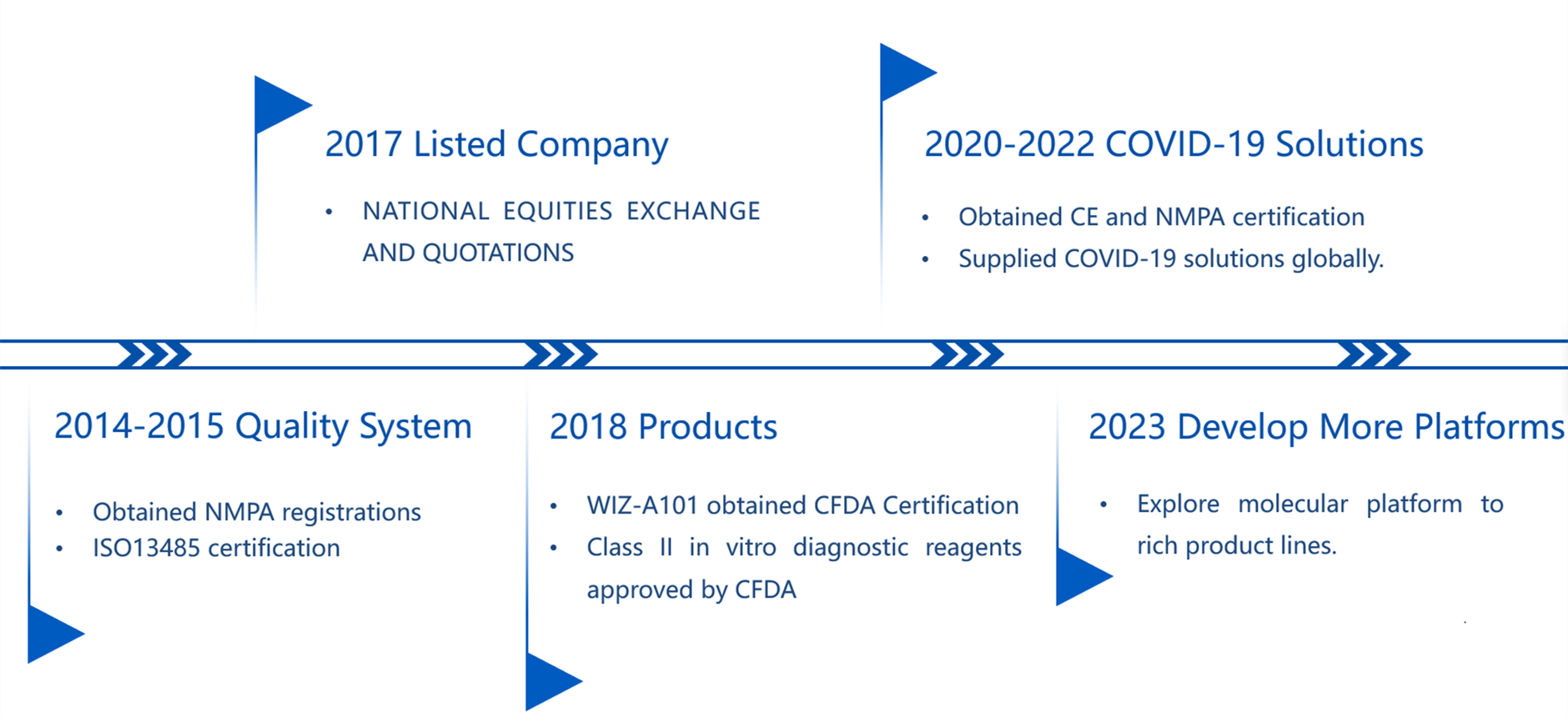
Milestone
Company Culture
Exhibition
Certificate










Partners




