Diagnostic Kit for antibody P24 antigen to Human Immunodeficiency Virus
1. INTEDED USE
This kit is suitable for the in vitro qualitative detection of human immunodeficiency virus antibody HIV (1/2) and antigen (p24) in human serum/plasma/whole blood samples as an aid in the diagnosis of human immunodeficiency virus infection. This kit provides results for the detection of human immunodeficiency virus antibodies HIV (1/2) and antigen (p24) only and the obtained result shall be analyzed in combination with other clinical information. It must only be used by healthcare professionals.
2. PRODUCT SPECIFICATION
| Model No. | HIV Ab/ P24 |
| Methodology | Colloidal Gold |
| Sample Type | serum/plasma/whole blood |
| Time to Result | 20-25 minutes. |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485 |


|
MAIN KIT COMPONENTS * Test device * Sample diluents * Disposable pipette * Instructions for Use |
PACKING
* 25 test /kit * Aluminum foil bag labeling * shrink wrap |
3.TEST METHOD
| 1 | Take the test device out of aluminum foil bag,place it on a flat tabletop and properly mark the sample. |
| 2 | For serum and plasma samples, take 2 drops and add them to the spiked wells; for whole blood samples, take 2 drops and add them to the spiked wells, then add 1 drop of sample diluent. |
| 3 | Result should be read within 20-25 minutes. Test result will be invalid after 25 minutes. |
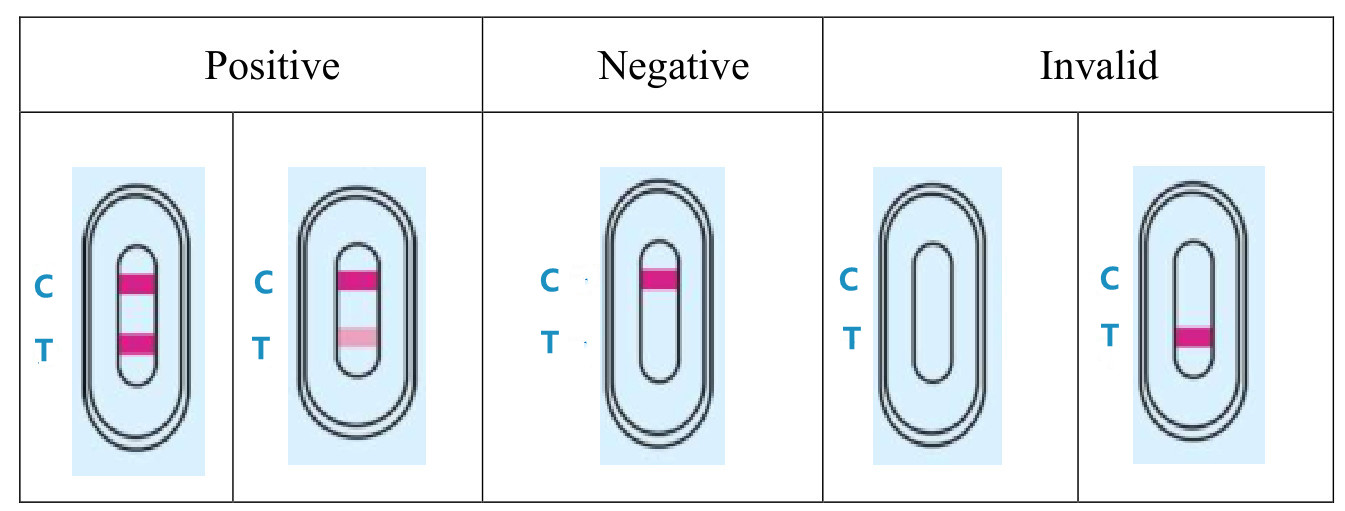
4.RESULT EVALUATION AND EXPLANATION
5.CLINICAL PERFORMANCE
The Baysen reagent test will be compared with the control reagent
| Baysen Result of HIV Ab/P24 | Test result of Reference reagent | Positive coincidence rate:99.07% (95%C.I. 94.94%~99.84%) Negative coincidence rate 99.55% (95%C.I.97.49%~99.92%) Total coincidence rate:99.39% (95%C.I.97.82%~99.83%) |
||
| Positive | Negative | Total | ||
| Positive | 107 | 1 | 108 | |
| Negative | 1 | 221 | 222 | |
| Total | 108 | 222 | 330 | |