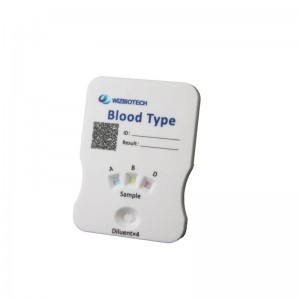
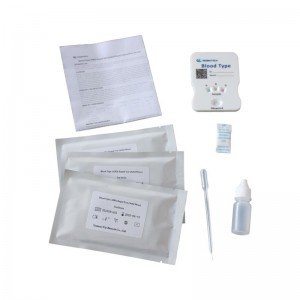
Blood type group ABD rapid test kit
1. INTEDED USE
This kit is suitable for the detection of ABD blood group system A/B antigen and Rh blood group system D antigen in human venous whole blood/fresh finger terminal blood/10% red cell suspensions in physiological saline and is not suitable for blood source screening.
2. PRODUCT SPECIFICATION
| Model No. | ABD |
| Methodology | Solid phase |
| Sample Type | whole blood |
| Time to Result | 3-5mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,CE,MHRA |


| MAIN KIT COMPONENTS: *Test device *solution *Instructions for Use |
Packing: *25 test /kit *Aluminum foil bag labeling *shrink wrap |
3.TEST METHOD
| 1 |
Before the test, the kit and the sample are taken out from the storage condition and balanced to room temperature(18°C~30°C) and mark it.
|
| 2 |
Tearing the packaging of the aluminum foil pouch, take out the test device and mark it, then place it horizontally on the test table.
|
| 3 |
Using a disposable pipette , add 1 drop (approximately 10 µL) of the sample to be tested to each well of A,B and D respectively. After the sample has been added, add 4 drops (approximately 200 µL) of sample rinse to the Diluent wells and start timing.
|
| 4 |
One minute after the sample rinse has been added, observe and record the results of the test, after 15 minutes the test result will be invalid.
|
| 5 |
Visual interpretation can be used in result interpretation.
|
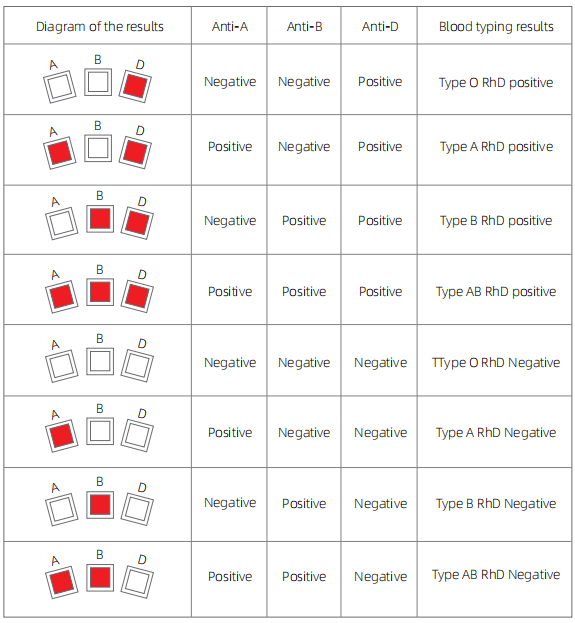
4.Result reading
5. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration