CTnI Cardiac Troponin I Test Kit For Acute Myocardial Infarction
1. INTEDED USE
This kit is intended for in vitro quantitative detection on the content of cardiac troponin I (cTnI) in human serum/plasma/whole blood sample and is intended for auxiliary diagnosis of myocardial infarction. This kit only provides cardiac troponin I (cTnI) test result, and the obtained result shall be analyzed in combination with other clinical information. This kit is for healthcare professionals.
2.PRODUCT SPECIFICATION
| Model No. | cTnI |
| Methdology : | Fluorescence Immunochromatographic Assay |
| Sample | Serum/Plasma/whole blood |
| Store | 2~30℃ |
| Time to test | result in 15 mins. |
| Shelf Life | 24 months |
| Certificate |
ISO13485,CE,MHRA |
3. COMPANY OVERVIEW

4. Exhibition

5. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration
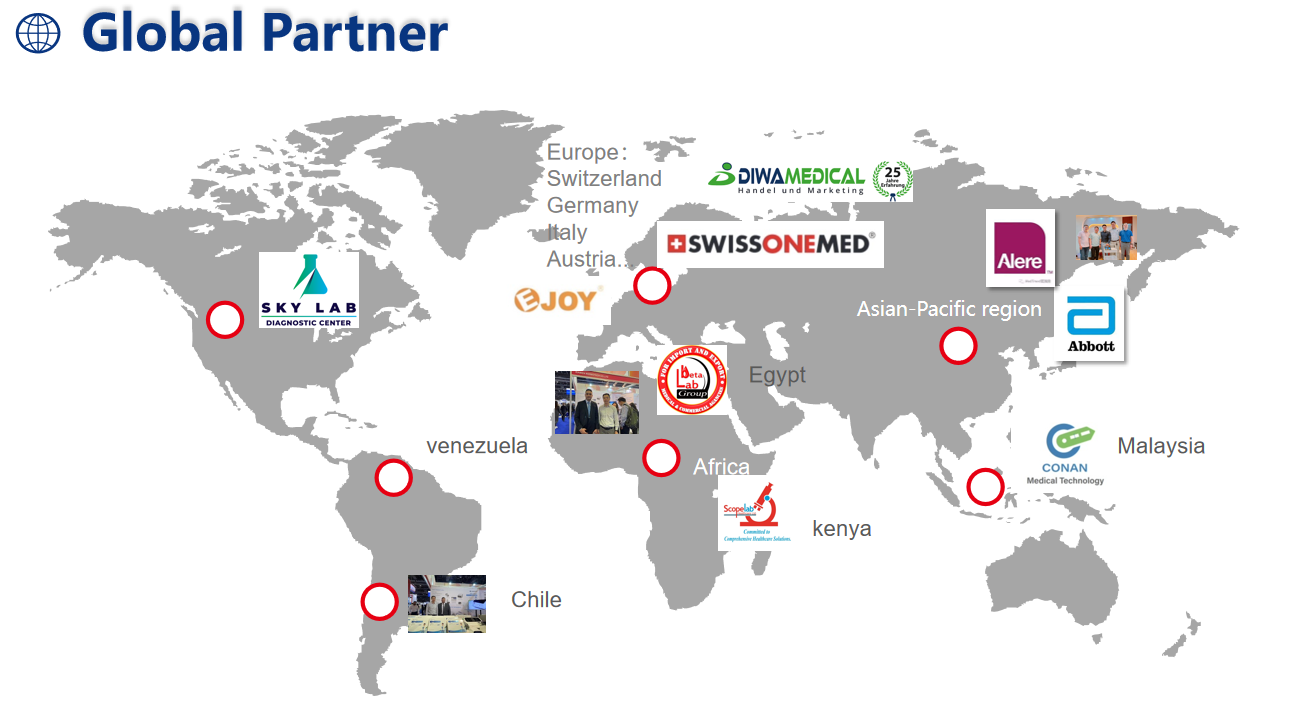
6. GLOBAL PARTNER