Hepatitis C Virus antibody infectious one step rapid test
1. INTEDED USE
This kit is suitable for the in vitro qualitative determination of hepatitis C virus in human serum/plasma/whole blood samples for the auxiliary diagnosis of hepatitis C virus infections. The results obtained should be analyzed in conjunction with other clinical information.
2. PRODUCT SPECIFICATION
| Model No. |
HCV
|
| Methodology | Colloidal Gold |
| Sample Type | serum,plasma,whole blood |
| Time to Result | 15-20mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485,MHRA |


| MAIN KIT COMPONENTS: *Test device *solution *Instructions for Use |
Packing: *20 test /kit *Aluminum foil bag labeling *shrink wrap |
3.TEST METHOD
| 1 | Before the test, the kit and the sample are taken out from the storage condition and balanced to room temperature and mark it. |
| 2 | Tearing the packaging of the aluminum foil pouch, take out the test device and mark it, then place it horizontally on the test table. |
| 3 | Open the reagent foil bag package, take out the reagent card, place it horizontally on the lab bench and mark the sample well |
| 4 | For serum and plasma samples, use a disposable pipete to take 2 drops and add them to the spiked wells; for whole blood samples, use a disposable pipete to take 2 drops and add them to the spiked wells, then add 1 drop of sample diluent. |
| 5 | Test results should be read within 15-20 minutes, if more than 20 minutes to read the results invalid. |
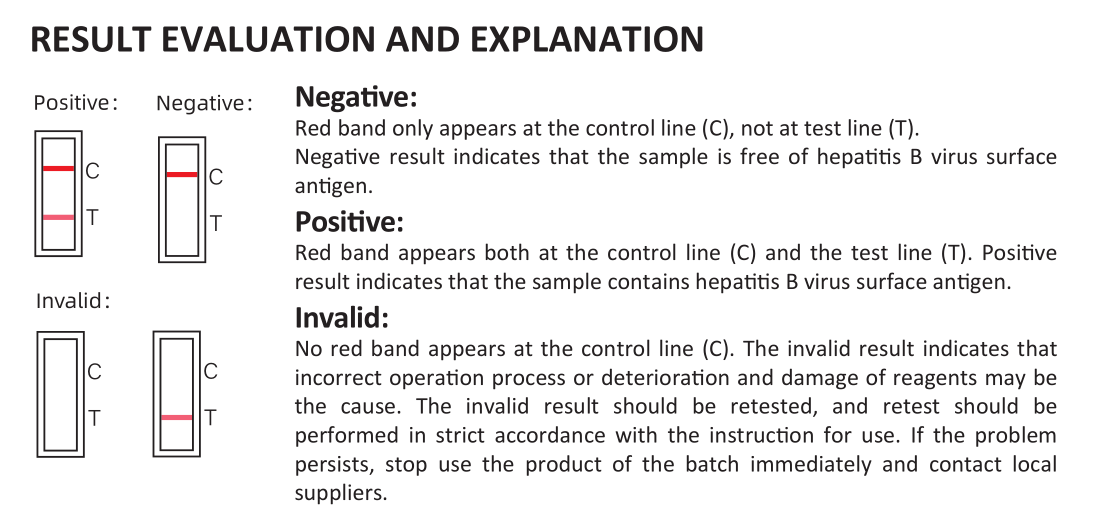
4.Result reading
5. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration