CE Approved Antigen to Adenoviruses rapid test kit
1. INTEDED USE
This kit is applicable to in vitro qualitative detection of adenovirus (AV) antigen that may exist in human stool sample, which’s suitable for auxiliary diagnosis of adenovirus infection of infantile diarrhea patients. This kit only provides adenovirus antigen test results, and results obtained shall be used in combination with other clinical information for analysis. It must only be used by healthcare professionals.
2. PRODUCT SPECIFICATION
| Model No. | AV |
| Methodology | Colloidal Gold |
| Sample Type | Faces |
| Time to Result | 10-15mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months |
| Certificate | ISO13485, CE Certificate, UCKA MHRA Certificate |

|
MAIN KIT COMPONENTS * Test device * Sample collection tube * Disposable pipette * Instructions for Use |
PACKING * 20 test /kit * Aluminum foil bag labeling * shrink wrap
|
3.TEST METHOD
| 1 | Use sampling tube for sample collection, thorough mixing, and dilution for later use. Use proof stick to take approx. 30mg of stool, place it in sampling tube loaded with sample diluent, screw the cap tightly, and thoroughly shake it for later use. |
| 2 | In case of thin stool of patients with diarrhea, use disposable pipette to pipette sample, and add 3 drops (approx.100μL) of sample dropwise to sampling tube, and thoroughly shake sample and sample diluent for later use. |
| 3 | Remove test device from aluminum foil pouch, lie it on a horizontal workbench, and do a good job in marking. |
| 4 | Discard first two drops of diluted sample, add 3 drops (approx. 100μL) of bubble-free diluted sample dropwise to well of test device vertically and slowly, and start counting time. |
| 5 | Interpret result within 10-15 minutes, and detection result is invalid after 15 minutes (see detailed results in result interpretation). |
4.RESULT EVALUATION AND EXPLANATION
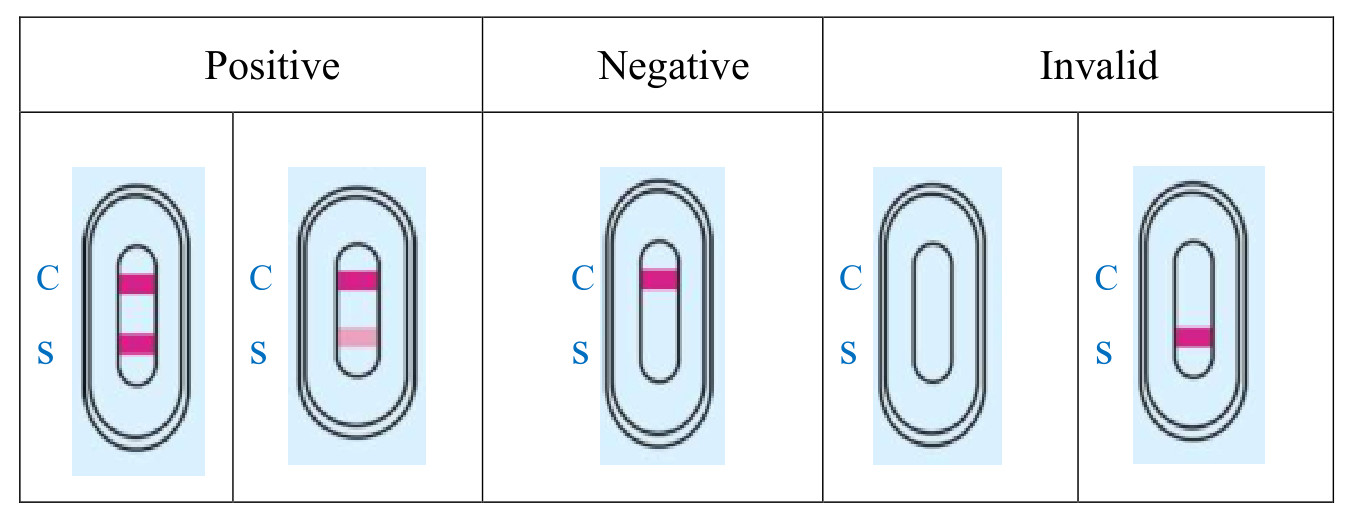
5.CLINICAL PERFORMANCE
Clinical performance of this product’s assessed through collection of 276 cases of clinical samples. Marketed kit of colloidal gold methods used as reference reagent, in comparison of Baysen reagent detection with reference reagent:
| Baysen Result of AV |
Test result of Reference reagent | Positive coincidence rate:98.54% (95%C.I. 94.83%~99.60%)) Negative coincidence rate:100.00% (95%C.I. 97.31%~100.00%) Total coincidence rate:99.28% (95%C.I. 97.40%~99.80%) |
||
| Positive | Negative | Total | ||
| Positive | 135 | 0 | 135 | |
| Negative | 2 | 139 | 141 | |
| Total | 137 | 139 | 276 | |
6. CERTIFICATE
* ISO System Certificate
* CE Certificate
* EU Registration
* UCKA MHRA Registration